5 Minute Read
Table of Contents
Beauty Standards: Now vs. Then
What Is a Breast Implant Removal Procedure?
Why Do People Have Their Implants Removed?
Allergan™ Textured Implant Recall
Unproven Link Between Breast Implants and General Ailments
What to Expect After a Breast Implant Removal
Does Dr. Hochstein Do Breast Implant Removal?
In 2018, more than 29,000 people underwent breast implant removal surgery.
Interestingly, that is 6 percent more than 2017 but 28 percent less than the year 2000.[1]
So what is causing the sudden rise in an otherwise decreasing trend of breast implant removals?
And why were breast implant removals on the decline anyway when breast augmentations are up a staggering 48 percent since 2000?[1]
The answer is actually pretty simple: surgeon experience and aesthetic preference.
Beauty Standards: Now vs. Then
During the ‘90s, celebrities like Pamela Anderson and Anna Nicole Smith helped make overly large breasts a popular fashion trend, causing many women to flock to their nearest plastic surgeon.
As most of these women would find out, the overly large aesthetic isn’t the most comfortable or practical, and, as the novelty of their new busts wore off, many would later have them removed.
Today, modern beauty standards place a much higher value on a curvy but overall balanced appearance.
As patients become more conservative with their implants, the need for a breast implant removal and a breast implant revision ultimately declines.
The experience pool available today is also much higher than in previous years, and this will continue to be the case year after year.
Medical students are now able to learn from surgeons with decades of experience in plastic surgery.
But external factors like textured implant recalls and the need to maintain breast implants after the initial surgery can be pushing the implant removal numbers higher than previous years.
What Is a Breast Implant Removal Procedure?
A breast implant removal surgery, sometimes referred to as a breast implant explantation or an explant surgery, is a procedure that removes the implant after a breast augmentation surgery.[2]
When the implant has a chance to set in the body, a layer of scar tissue forms around the implant that helps bind it in place.
More breast revision before and after images are available on Dr. Hochstein’s gallery.
When the implant needs to be removed, it can be explanted in many small sections, or the implant and scar tissue can be removed in one piece with a technique called en bloc capsulectomy.
The en bloc technique prevents the implant filling material from leaking into the body since the intact scar tissue completely encapsulates the implant as it is removed.
Why Do People Have Their Implants Removed?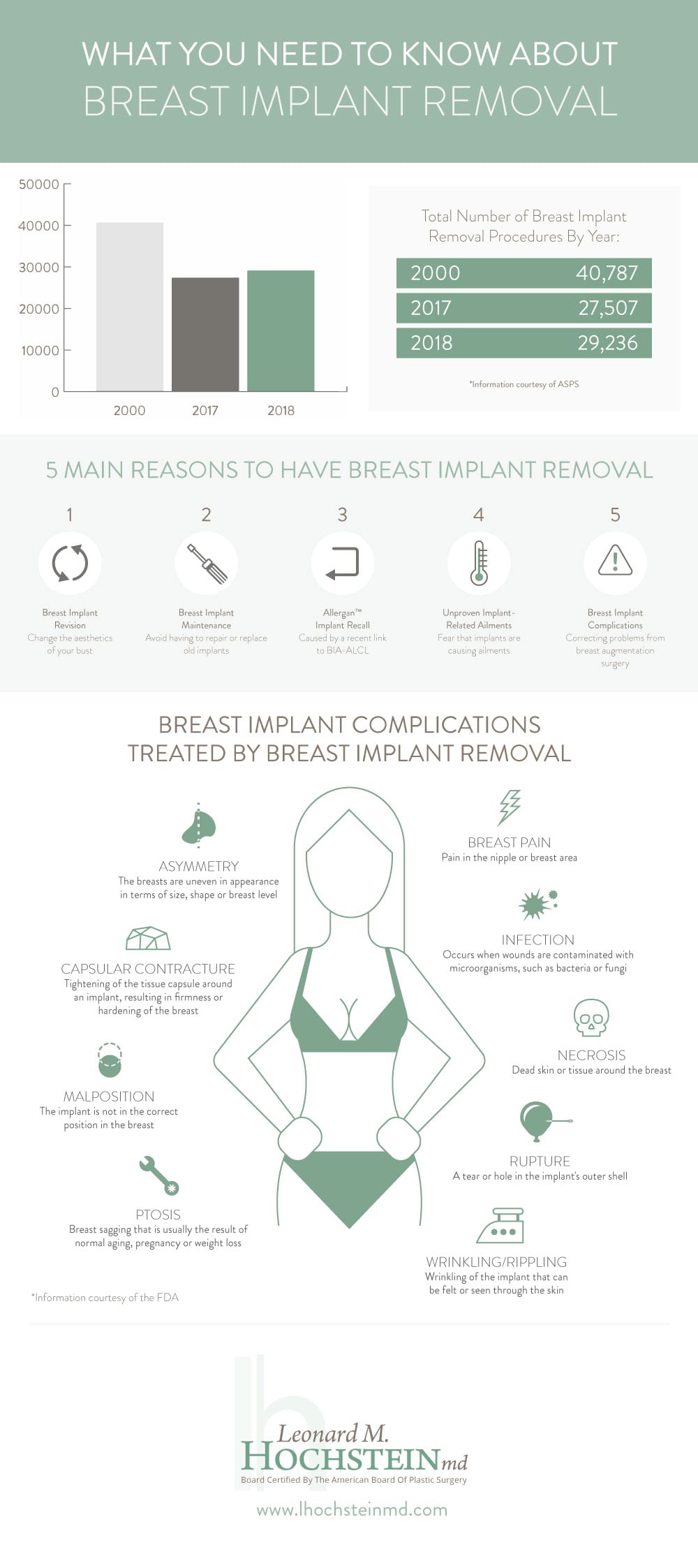
There are 5 main reasons why a person would consider a breast implant removal procedure:
1) Breast Implant Revision
When you are in your 20s, you have a very different set of goals and priorities than when you are in your 40s and 50s.
Naturally, the large bust you adored in your 20s might not fit the lifestyle you are hoping to live in your later years.
Or maybe you are still 20, but you decided to be more conservative when you were increasing the size of your breasts and now you find yourself underwhelmed with the results.
Whatever the case may be, preferences can change, and breast implant revision is the best way to change the size, shape, or projection of a breast augmentation that did not leave you with the body you desired.
2) Breast Implant Maintenance
Many people believe that a breast augmentation is a one-and-done procedure. The truth is that breast implants are not designed to last a lifetime and, at some point, implant maintenance or an additional surgery may be needed.
This sometimes means having to replace an entire implant and paying for that surgery and new implant.
Rather than continue to drop money into the problem, some people opt to remove their breast implants once they begin to experience complications.
3) Allergan™ Textured Implant Recall
Some of the biggest news of 2019 in the plastic surgery industry was the total recall of certain Allergan™ textured breast implants.[3]
The reason behind the recall was a link found between the Allergan™ textured implants and a condition called breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
Here is a list from the Federal Food and Drug Administration of exactly which textured Allergan™ products were recalled[4]:
- Allergan™ Natrelle Saline-Filled Textured Breast Implants
- Allergan™ Natrelle Silicone-Filled Textured Breast Implants
- Allergan™ Natrelle® 410 Highly Cohesive Anatomically Shaped Silicone-Filled Textured Breast Implants
- Allergan™ Natrelle 133 Plus Tissue Expander
- Allergan™ Natrelle 133 Tissue Expander With Suture Tabs
Allergan™ was asked to recall the implants by the FDA, who found that out of 573 patients observed with BIA-ALCL, 481 of them had Allergan™ textured implants.[5]
If you have questions about these implants or BIA-ALCL, you can visit the FDA’s FAQ page for information.
A side by side comparison of a textured implant (left) and a smooth implant (right). Allergan™ recalled multiple textured products after a link with BIA-ALCL, a type of lymphoma, was found by the Food and Drug Administration.
It is important to note, however, that while the FDA did request the recall, they are recommending that only patients who are showing symptoms of BIA-ALCL consider a breast implant removal.[5]
4) Unproven Link Between Breast Implants and General Ailments
The term “breast implant illness” was created by groups of women who began experiencing a host of different symptoms at some point after their breast augmentations. These women believed that their symptoms were caused by their implants. However, there is no data or research that links breast implants to breast implant illness. This is simply a case of correlation rather than causation.
What Are the Claimed Symptoms?
The symptoms claimed to be related to implants are common ailments that can stem from a variety of causes. Here is a list of symptoms that women associate with their implants: fatigue, chest pain, hair loss, headaches, chills, sensitivity to light, rash, body odor, anxiety, brain fog, sleep disorders, depression, neurologic issues, and hormonal issues.[6]
Currently, breast implant illness is not recognized by the greater medical community as a real condition since implants have not been proven to cause any disease or illness. However, many women self-identify with this illness and choose to remove their implants for peace of mind.
5) Breast Implant Complications
While a breast augmentation is considered to be a highly safe and routine procedure, there is always the possibility that a complication or side effect will develop. Some of the most common complications include:
- Capsular contracture
- Implant rupture
- BIA-ALCL
- Asymmetry
- Breast pain
- Capsular contracture
- Infection
- Implant malposition
- Necrosis
- Ptosis rupture
- Breast revision
- Breast pain
- Changes in nipple sensation
What to Expect After a Breast Implant Removal
The incisions that accompany a breast implant removal are very similar to those of a breast augmentation, which means the recoveries will be very similar—roughly 2 weeks before you are able to return to work and 6 to 8 weeks before you can resume strenuous exercise.
Since the skin and tissue will be stretched out from the breast implant, you may notice your breasts sag more than they did previously.
Combining your breast implant removal with a breast lift can trim away that excess skin to preserve the shape of your smaller breast size.
Does Dr. Hochstein Do Breast Implant Removal?
Dr. Hochstein performs both a breast implant removal with the en bloc technique and a breast revision for patients who are no longer happy with their breast implants.
View this post on Instagram🤩🤩 3 month post op breast revision (breast lift and implant exchange), 415 MP
More breast revision before and after images are available on Dr. Hochstein’s gallery.
If you would like more information about breast implant removal, contact Dr. Hochstein’s Miami, Florida, office at 305-931-3338.
[1] https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics
[2] https://www.plasticsurgery.org/cosmetic-procedures/breast-implant-removal
[3] https://www.fda.gov/medical-devices/medical-device-recalls/allergan-recalls
[4] https://www.fda.gov/medical-devices/medical-device-recalls/allergan-recalls-natrelle-biocell
[5] https://www.fda.gov/medical-devices/
[6] https://www.surgery.org/sites/default/files/downloads/BII-Talking-Points-FINAL-1.15.19.pdf